Abstract
INTRODUCTION
Frailty in patients undergoing hematopoietic cell transplantation (HCT) has a negative impact on survival. The inclusion of frailty evaluations before HCT is highly recommended; however, there is no consensus about the best methodology to evaluate this syndrome.
In 2018 our center started a Frailty and Functionality program that involves regularly collecting information on the following eight indices in patients referred for allogeneic HCT (Salas et al., 2021): Clinical Frailty Scale (CFS), Instrumental Activities of Daily Living (IADL) test, Timed up and Go Test (TUGT), Grip Strength (GS), Self-Health Rated questionnaire (SHR-Q), Fall test, albumin (Alb), and C - reactive protein (CRP). With the recorded measurements of the eight indices and the corresponding validating process, we propose a HCT Frailty Scale. This scale is specifically designed to identify fit, pre-frail, and frail candidates for alloHCT, from better to worst probability of post-transplant survival.
METHODS
Between June 2018 and December 2020, 338 adults underwent alloHCT at our Institution. Frailty syndrome was evaluated prospectively in all patients at first consultation, using existing resources, after informed consent. It included measurement of the eight indices. The median time to evaluate was 5-6 minutes. Each index was given a value of 0 if normal or 1 (abnormal). Complete data were available for 298 patients that were finally included in the analysis. With this data the HCT Frailty Scale was elaborated as follows.
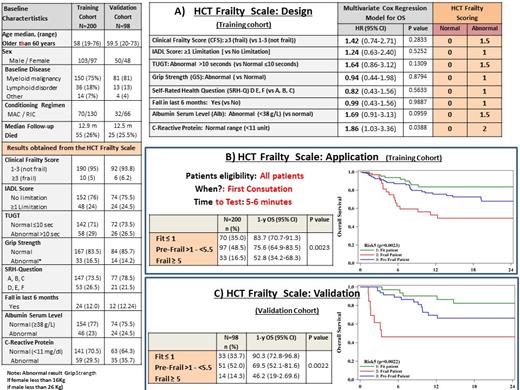
The study cohort was split in two groups, a training cohort with 2/3 (N=200) of the patients, and a validation cohort of 1/3 (N=98), proportional to death outcomes. With the data from the training group we estimated a multivariable Cox model with overall survival (OS) as dependent variable, and the eight referenced indices as explanatory variables. Any normal result was scored 0, and based on the estimated HR coefficient from the Cox model, a proportional weight score was given to each respective index variable in the calculation of the composite HCT Frailty Scale score. The HCT Frailty index was calculated using the following formula: 1.5 *CFS, +1*IADL, +1*GS, + 1.5*TUGT, + 1*SHR-Q, +1*Falls-Test, + 1.5 *Alb, + 2*CRP. As a result, the HCT Frailty Scale goes from 0 to 10.5. The values of the scale were grouped to determine the following three groups of patients: (a) Fit patent: scale score ≤1; (b) Pre-Frail patient: 1< scale score < 5.5; (c) Frail patient: scale score 5.5 (Figure 1).
RESULTS
Baseline characteristics of the training and validation cohort are shown in Figure 1. Of the 200 patients included in the training cohort, the median age was 58 years (range 19-76 years); 29 (15.85%) had a KPS between 70-80%; and 56 (30.11%) a HCT-CI >3.
The elaborated HCT Frailty Scale classified the 200 patients as: (a) 70 (35%) fit patients with an estimated 1-y OS of 83.7%; (b) 97 (48.5%) pre-frail patients with a predicted 1-y OS of 75.6%; and (c) 33 (16.5%) frail patients with an estimated 1-y OS of 52.8%. Of the 33 frail patients, 54.8% had a KPS between 90-100% and 48.5% had an HCT-CI <3 and of the 70 fit patients, 4.8% had a KPS between 70-80% and 24.2% had an HCT-CI ≥ 3. These differences support the hypothesis that frailty does not necessarily correlate with performance and comorbidities. The predictive ability of the HCT Frailty Scale was validated in 98 patients included in the validation cohort. This scale identified (a) 33 (33.7%) fit patients with an expected 1-y OS of 90.3%, (b) 51 (52%) pre-frail patients with an expected 1-y OS of 69.5%, and (c) 14 (14%) frail patients with an estimated 1y OS of 46.2% (Figure 1).
CONCLUSION
The HCT Frailty Evaluation Scale has been specifically designed to be applied in routine clinical practice and to patients across all ages. The scale ranges from 0 to 10.5 and the score value is calculated as the weighted sum of values of eight indexes evaluated at first consultation. The proposed scale should be of utility to identify frail and pre-frail patients that may benefit from appropriate counselling pre-transplant and individualized interventions to reverse frailty syndrome prior to alloHCT.
Law: Novartis: Consultancy; Actinium Pharmaceuticals: Research Funding. Kim: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Paladin: Honoraria, Research Funding; Bristol-Meier Squibb: Research Funding; Pfizer: Honoraria, Research Funding. Lipton: Bristol Myers Squibb, Ariad, Pfizer, Novartis: Consultancy, Research Funding. Mattsson: MattssonAB medical: Current Employment, Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal